DISRUPTING THE GLOBAL PHARMACEUTICAL INDUSTRY WITH CANNABINOID-BASED MEDICINE
Introduction to Avicanna, an international commercial-stage biopharmaceutical company with a focus on commercial products and pharmaceutical pipeline
- Canadian biopharmaceutical company with disruptive on cannabinoid based medical and pharmaceutical products
- Commercial stage with growing revenues across 16 international markets
- Industry leading scientific platform and IP portfolio established in Johnson and Johnson incubator since 2017
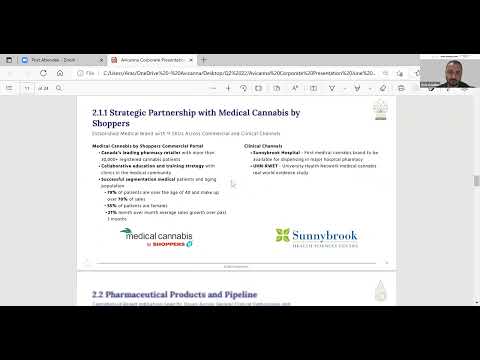
- 30+ commercial products including leading brand in Canada’s leading pharmacy chain and an extensive pharmaceutical pipeline
- Organic and low cost vertical integration and supply chain business in Colombia
Event Recording
DISRUPTING THE GLOBAL PHARMACEUTICAL INDUSTRY WITH CANNABINOID-BASED MEDICINE
Speaker
Aras Azadian
- Title
- Chief Executive Officer @
- Company
- Avicanna
- Role
- Speaker
Utilizing his extensive senior management experience in both financial and bio-technology sectors, Aras co-founded Avicanna with the vision of establishing a bio-pharmaceutical company with a strict focus on medical and pharmaceutical applications of cannabinoids. His expertise and experience in the biotechnology industry have been integral to Avicanna’s thought leadership pertaining to R&D and clinical development. Since 2016 Aras has successfully led a team of executives, scientists, and medical professionals across several countries with the vision of vertical integration and a strong company focus on quality controls, scientific vigour and competitive advantages. Aras holds a Bachelor of Economics degree from York University and an International master’s in management degree from EADA Business School in Barcelona, Spain.
About
Avicanna
Avicanna is a commercial-stage international biopharmaceutical company focused on the advancement and commercialization of evidence-based cannabinoid-based products for the global medical and pharmaceutical market segments. Avicanna has an established scientific platform including R&D and clinical development that has led to the commercialization of more than thirty products across various market segments:
Medical Cannabis & Wellness Products: Marketed under the RHO Phyto™ brand these medical and wellness products are a line of pharmaceutical-grade cannabinoid products containing varying ratios of cannabidiol (“CBD”) and tetrahydrocannabinol (“THC”). The product portfolio contains a full formulary of products including oral, sublingual, topical, and transdermal deliveries that have controlled dosing, enhanced absorption and stability studies supported by pre-clinical data. The formulary is marketed with consumer, patient and medical-community education and training.
Pharmaceutical Pipeline: Leveraging Avicanna’s scientific platform, vertical integration, and real-world evidence, Avicanna has created a pipeline of patent-pending drug candidates that are indication-specific and in various stages of clinical development and commercialization. These cannabinoid-based drug candidates look to address unmet medical needs in the areas of dermatology, chronic pain, and various neurological disorders. Avicanna’s first pharmaceutical preparation (Trunerox™) is in the drug registration stage in South America.